
Co2 And Ph??? Probes And Solenoids.
#1

Posted 02 February 2012 - 11:21 PM
A CO2 solenoid will control the amount of CO2 that enters the tank based on the given value of PH.
We aim for 30ppm CO2 and a PH of 6.6?
If the PH drops below 6.6 the solenoid will close. Must we then raise the PH so the CO2 solenoid reopens or will the PH rise naturally due to the lack of CO2 and then the solenoid reopen?
What if i have had CO2 pumping into my tank and my PH reaches the cut off of 6.6 and i only have 15ppm of CO2? What do i need to do to have the CO2 flow continue until i reach my target ppm without running the risk of lowering my PH below 6.6?
Thanks.
#2

Posted 02 February 2012 - 11:30 PM
A controller/monitor turns on/off solenoid depending on ph.
Pop in to aquotix and talk to Ollie. He knows his stuff :3
#3

Posted 03 February 2012 - 12:25 AM
A CO2 solenoid will control the amount of CO2 that enters the tank based on the given value of PH.
We aim for 30ppm CO2 and a PH of 6.6?
If the PH drops below 6.6 the solenoid will close. Must we then raise the PH so the CO2 solenoid reopens or will the PH rise naturally due to the lack of CO2 and then the solenoid reopen?
What if i have had CO2 pumping into my tank and my PH reaches the cut off of 6.6 and i only have 15ppm of CO2? What do i need to do to have the CO2 flow continue until i reach my target ppm without running the risk of lowering my PH below 6.6?
Thanks.
The scenario you postulate will not occur if you have established the correct alkalinity (kH). I suggest you do some research: there are numerous sites explaining the relationship between carbon dioxide levels and alkalinity.
Syd.
#4

Posted 03 February 2012 - 05:44 PM

The solenoid allows you to turn the CO2 on or off without turning it off at the bottle ( I use mine on a timer with the lights )
You can also get a pH controller

This monitors the pH level in your tank and then in turn , allows or cuts the power to your solenoid , which then adjust the pH
Hope that helps
Graeme
#5

Posted 03 February 2012 - 06:47 PM
ALL staff at AQUOTIXS Know there stuff.
Lets start off with KH (CO3 or carbonates):
KH is basicly the buffering ability of your water. The higher the KH, The more your pH is resistant to change.
For example : If I have a KH of 0, my pH tends to swing wildly when doing water changes, but if my KH is 7' Dg I will have a very stable pH, although some fish do lot like it this high.
CO2 gas injected into the aquarium will form carbonic acid (H2CO3) which will decrease your pH.
Obviously the Higher your KH the more CO2 you will need to decrease it to your desirable pH there is a calculable relationship for this.
Here is commercially available CO2 saturation chart, lets take a look at this relationship:
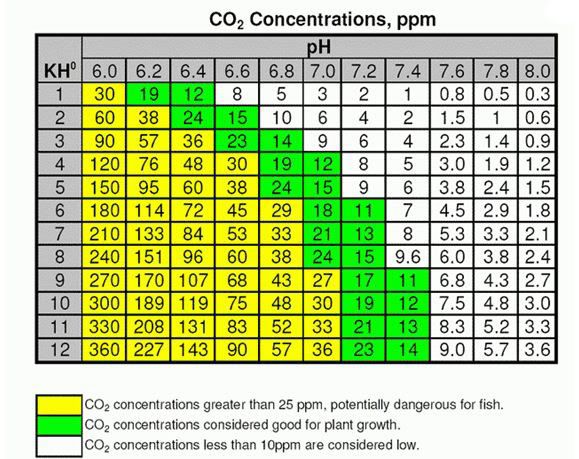
As I said before, with high light you need high CO2, ferts,macro and trace elements.
With Low light, you only need to give the plants up to the level at which they are limited : Light - The Ultimate determining factor of growth.
Less light > less CO2, Less ferts, Less Growth....
High light > High Co2, High ferts, High rate of Growth...
In my personal aquarium I have many plants and VERY strong light, with an Intensive fert Program because I want very fast growth.
So I Aim for a CO2 level of 30ppm (very high)- This in the table IS a pH of 6.8 and a KH of 6....which I maintain.
I just set my pH controller to 6.8 and it will disperse ONLY enough to maintain the set pH wheather plants absorb it or not.
A pH controller is a usefully tool, that will disperse the Maximum amount of CO2 to maintain A constant CO2 level...In other words "Set and forget".
And a pH controller will adjust to your plant load, add more plants and more CO2 will be dispersed, Sell the whole tank full of plants and hardly any will come out.
You also don't have to turn it off at night or worry about messing up the bubble count and killing your fish.
As long as you maintain the Correct KH and regularly Re-calibrate your probe its all to easy.
For a tank like yours, with Medium-light (2x 5ft HO ?) you would want to aim for a CO2 of say 15-20ppm, there are many different combinations for this above, but i wouldn't recommend a pH lower than 6.4.
Rovik.
#6

Posted 03 February 2012 - 09:30 PM
one point to consider though, particularly with the use of Aquasoils now-a-days....
If you look at the chart above - you will notice an assumed pH at a known KH with very little CO2.... this is assuming that there are no other acids in play in the tank at the time (such as the organic acids released daily by aquasoils) - what I am trying to say is, sometimes you can have a KH of 2, but a pH of 6.4 BEFORE CO2 introduction.... this does not mean you have 24ppm of CO2... so sometimes the chart is misleading.... you should check the pH of the tank before adding CO2 (after a water change is good) and this is considered your "BASE pH"... then add CO2 until the required drop in pH is obtained to give you approx 20-30ppm of CO2 in the known KH level band..... (it is hard to explain, best if I can explain it in person....) and remember, the base pH will change over a week or two, so it is good to compare the base pH weekly to see how your tank behaves.
Use the chart as a guide, not as gospel... as there are other things in play at times that may affect the outcome.
#7

Posted 03 February 2012 - 09:58 PM
Thank you very much.
One last question though.....KH?
How are KH levels maintained?
Does the Estimative Index take care of this?
I got some metal halides and i plan to use them and CO2 in the new house so i guess ill be having a high tech tank after all.
Edited by kassysimon, 03 February 2012 - 11:02 PM.
#8

Posted 04 February 2012 - 10:26 AM
Edited by Mr_docfish, 04 February 2012 - 10:26 AM.
#9

Posted 04 February 2012 - 02:03 PM
Hello Oliver,
I presume this is potassium bicarbonate and used because it is more soluble than calcium/magnesium carbonates.
This would allow you to raise the kH more quickly, but are there any other advantages?
The extra calcium and magnesium is generally beneficial to plants.
Cheers
Brett
#10

Posted 04 February 2012 - 09:13 PM
I also supply KOH (Potassium Hydroxide) for increasing the pH without increasing the KH long term (great for neutralizing the organic acids in aquasoils without any unwanted by-products) but it is to be used in very small volumes.... this is what I use to help me increase the CO2 injection without having to add any carbonates or bicarbonates.
EDIT: (see sydad's post below)
Edited by Mr_docfish, 05 February 2012 - 09:37 PM.
#11

Posted 04 February 2012 - 10:03 PM
What i plan to do is have two reservoirs, one for the macro and one for the micros and have those reservoirs, each connected to a pump, controlled by a timer (Profilux probably) that will pump the required amount of solution into the tank as required.
Ill be making the solution based on this EI website http://calc.petalphile.com/en/
I want to make one solution that will have KNO3, KH2PO4, MgSO4, K2SO4 (Dave from Aqua Green suggested mix) and another that will have the chelated iron mix, also from Dave.
An Eheim 300 internal pump will need to run for 6 seconds to pump 500ml of solution. Based on it pumping 300L ph. (300L ph / 60m = 5L pm / 60s = .083L ps X 6 seconds = .500L) plus the required time required to make up for the reduced flow due to the DuCV. A few test runs will resolve this.
I have already hard plumbed-in two FX5's. With-in that plumbing is a DIY CO2 reactor which is just a piece of 100mm PVC pipe capped at both ends and filled with bio balls that the filters return line runs through. The CO2 is injected into the bottom of this. I then have a DIY external heater housing which is another piece of 100mm PVC capped at both ends with two 300w Jager heaters inserted into it that also has the return line from the two filters running through it. This keeps everything out of sight. I plan to just connect the pump from the solutions into the return line plumbing via a DuCV.
Anyways, what i dont know is whether the solutions will react or something whilst in the reservoir. Can this be done?
KH will be added manually as required.
Thanks
Simon.
Edited by kassysimon, 04 February 2012 - 10:04 PM.
#12

Posted 04 February 2012 - 11:11 PM
I want to make one solution that will have KNO3, KH2PO4, MgSO4, K2SO4
KH will be added manually as required.
Simon.
These should be OK to mix; All potassium salts are soluble in water so your safe there, Just don't go adding calcium into that mix as Calcium is relatively high in the reactivity series and may potentially precipitate out into Calcium Phosphate (In-soluble).
MicroNutrient mixes should be made in a solution of Distilled water if possible for the same reasons, They only last about 2 weeks so need to be re-done after this time if possible.
The EI method is NOT a be all and end all method.
I don't use it and I have wonderful results.
Flush...Refill and dose is time consuming. So it suits people like Tom Barr just fine, He gets paid to do it.
I have worked out a Hybrid method for my individual tank needs/plant/fish load, because in an aquarium, things change all the time.
I heard of a guy who DID NOT test his water for 10 years on his planted tank because "he used" the EI method and knew exactly what was in his tank.
If your not monitoring your nutrient levels After 10 years I guarantee that something is no what you Estimated it to be.
And that is what the Estimative index is.....Just an Estimate.
Its a great method if you have the time/money/resources..
But I prefer to test my water and add what I need...Eventually you get into a routine and you know That each water change you Need to add something.
I always test after a water change anyway (3h later).
For example if I a have a Sh*tload of fish in my tank for whatever reason they are eating A LOT, I have an excess of PO4 and I continue with my normal 50% water changes once a week and dose with my normal say 1ppm of PO4, in a very short time I will have an excess of PO4 Which have been linked to algae - (Arguable, but for another time
So I have to say on top of this by testing my PO4 levels and watch my feed, the next batch of Ferts I do I will reduce the amount of PO4.
That's why it is always easier to Keep the fert as dry salts instead solution because you can alter your fert regime with a quick water change and dosing the regular amount including or excluding things you lack or have to much of.
This is just one scenario out of possible hundreds.
The EI will be effective at the same dose rate if you are eliminating all of the nutrients (Being absorbed by plants) within your water change periods. (usually weekly)
This requires a lot of tinkering on your part to stay on top of your levels but never the less, Effective.
Hope this helps Simon
Rovik.
#13

Posted 05 February 2012 - 12:16 AM
KNO3 = potassium Nitrate.
KH2PO4 = monopotassium Phosphate
MgSO4 = Magnesium Sulphate
K2SO4 = Potassium Sulphate
When and why would i add Calcium? It can be added to the tank as required though?
I have built an auto change over system that i can control with my iPhone and is directly plumbed into my drainage system for emptying the tank and also plumbed into my mains pressure for filling, run through a tempering valve so the water is added to the tank at 26°. That coupled with my auto dosing method will also save alot of time as i have designed a system that will add the correct amount of dry ferts and DI water to my reservoirs automatically so all i need to do is top up my dry fert housing and test and prune. CO2 will also be automatic.
This will save me alot of time so i can therefore spend it on the EI and testing.
I will still be testing my water even though i will be using EI. I like to know whats happening. That way i can purpose dose too.
Thanks again.
Once fully set p (about 6 months) i will post a pic of the full system.
Thanks for your help.
Edited by kassysimon, 05 February 2012 - 12:21 AM.
#14

Posted 05 February 2012 - 12:31 AM
I also supply KOH (Potassium Hydroxide) for increasing the pH without increasing the KH long term (great for neutralizing the organic acids in aquasoils without any unwanted by-products) but it is to be used in very small volumes.... this is what I use to help me increase the CO2 injection without having to add any carbonates or bicarbonates.
Um Ollie, calcium and magnesium bicarbonates actually don't exist in the dry state (so cannot be purchased for any cost in this form); only in very dilute solution, and in the presence of dissolved carbon dioxide. As for the potassium hydroxide, it is rapidly converted to the carbonate and then bicarbonate in the presence of excess carbon dioxide, so the kH increases regardless unless only enough KOH is used to neutralise the organic acids ®. The K® so formed will eventually break down to release the potassium moeity which will probably contribute to kH increase unless absorbed by plants.
Syd.
Edited by sydad, 05 February 2012 - 12:34 AM.
#15

Posted 05 February 2012 - 09:44 AM
Surely thats the point of EI, you replace ALL the water every week so you do know what is in it .
Cheers
Brett
#16

Posted 05 February 2012 - 09:13 PM
Syd.
ahhh yes.... you are right Syd - I was thinking of Ca and Mg carbonates, not bicarbonates..... my bad - too much alcohol at that time of night....
The idea of adding KOH was just as you mentioned (and I noted also - "to be used in very small volumes") - to neutralize the organic acids, allowing more CO2 to be added without dropping the pH any further.... and the small amount of K added will add to the K requirement of the plants, unless one already adds heaps of K in other forms.....
Thanks for picking me up on the bicarbonate thing... good to have someone like you to pick
Cheers Syd - you da man....
Oliver
Edited by Mr_docfish, 05 February 2012 - 09:14 PM.
#17

Posted 05 February 2012 - 09:22 PM
Except for how to spell their trading name
#18

Posted 05 February 2012 - 09:33 PM
Surely thats the point of EI, you replace ALL the water every week so you do know what is in it .
Cheers
Brett
Not testing the water leads one into problems, and make assumptions as to how to tweak the dosing to suit any changes required....
For example - if you used a particular dosing system for a number of months, and then decided you wanted to try different range of plants, then the dosing system has to change to suit the new types of plants and the change in plant density..... how much PO4 can a tank full of plants consume in between water changes? Testing water will assist in advising the plant keeper as to what to increase and what to decrease or even omit as required. If you feel ill, went to the doctor and told him/her what you do every day and what you eat every day, will they still prescribe you with a treatment without doing even the simplest of tests? Observing with ones eyes can only determine issues AFTER the problem crops up... water testing can identify issues BEFORE they occur.
Before you make a solution of ferts, test the water you intend to use - and test the aquarium before you add ferts to see what is already in there (aquasoils can add sufficient N and P in some cases - but only for a limited time, after which things change, and dosing has to change with it) check to see if you need to add PO4, or NO3 if you are using aquasoils. Check if you need Ca if you like SOR (Ca poor tap water).
Also, a week after dosing, check the levels to see what is being used up quickly, so you can add more for the next mix.... and find out what is not being used, or even increasing, and reduce that.
I even went to the extreme of buying a K test kit to prove Tom Barr wrong on the K vs Ca uptake issue..... I was right, but he did not want to hear it - he does not believe in test kits.... one day I will send him plants like the hard to grow Aussie native - Aponogeton bullosus, you cant grow it under his EI method with the levels of PO4 and K he recommends.... but simple to grow stem plants just love it - and some types of algae love it too - but that is another bone of contention..... every tank and every plant is different, good luck in finding out what works for you - it is fun to play around and get results.
#19

Posted 05 February 2012 - 09:37 PM
Website:
My spelling: AQUOTIXS ?
its not aquatics...its Aquotix.....
#20

Posted 05 February 2012 - 09:40 PM
I take it back, sorry, it's just your grammar that's bad!
And you posted at 6:47pm
I'll let this all go back on topic now, continue gents
0 user(s) are reading this topic
0 members, 0 guests, 0 anonymous users













